| All of the sections listed in your report before the Methylation section, will have an impact on the functioning of your Methylation Pathway.
If you’ve already optimised your Gastro-Intestinal health, lowered your inflammation, addressed hidden infections and immune system function, optimised all aspects of your detoxification and mitochondrial function, then you have already addressed your Methylation Pathway in a very significant way. Now you can start being more specific with the genes and cycles you need to support, going forward. You may need to start addressing methylation, to some extent, while addressing your GI health, if your Homocysteine is high at that stage. Please note that many substances commonly used to address the methylation pathway, may have an impact on the levels of your neurotransmitters. Your specific genotypes, listed under the NEUROTRANSMITTERS section, may influence how you react to these substances, as far as mood and cognitive processing are concerned. |
This article is part of the educational content offered with the interpretation of your DNA results.
METHYLATION OVERVIEW
Most articles about methylation we’ve come across from Naturopathic sources, focus on this topic in a very generalised manner, often, by bullet-pointing the many benefits of addressing methylation and what nutritional or supplementary interventions may ‘aid’ in that.
When dealing with the topic of Methylation, we need to make sure that we draw a distinction between how this term is being used in the scientific literature and how it often tends to be misused in communities focusing on nutrigenomics.
There are two ways in which we can talk about methylation:
I. DNA METHYLATION
A process involving DNA modification that influences the level of genetic expression.
The dynamics by which methyl groups are moved along and attached to certain regions of DNA, in order to modify the shape and ‘behaviour’ of the DNA structures, are all wrapped up under the term of DNA Methylation.
When a CpG island in the promoter region of a gene is methylated, expression of the gene is repressed (it is turned off).
This is the aspect of methylation that the mainstream medical science and Epigenetic researchers focus on, the most. It has a very promising future in terms of drug and pharmaceutical development, cancer therapies, as well as anti-aging medicine applications.
These are also the epigenetic patterns that the Naturopathic practitioners like to talk about, but rarely go into much effort to explain how they tie into the proposed therapeutic regimens (risk vs. benefits).
Because the key mechanisms, playing the main role here, are very rarely discussed between naturopathic practitioners and their clients, we would like our readers to take some time to get familiar with these terms:
1) DNA Hyper (overactive) methylation
Over-methylated DNA can change gene expression by disabling its ability to manufacture enzymes or proteins. These changes in methylation patterns can lead to the development of diseases like cancer (Ref.) and we will touch again on this issue further down, in the article.
It can also contribute to immune system dysfunction, brain health problems and faster progression of the aging process. (Ref.)
2) DNA hypo (underactive) methylation
In this scenario, there aren’t enough methyl groups or the DNA methylation process is slowed down, which can lead to unstable DNA and cause mutations, leading to cellular transformation. (Ref.)
Both hyper and hypo – methylation can therefore be implicated in cancer development and progression, although there are specific gene targeting therapies against cancer which use hypomethylation as a way to slow down cancer growth. This can be beneficial for cancer in the short-term, but it may also speed up cancer growth (Ref, Ref. )
Underactive methylation has also been shown to have its contribution towards diseases linked to immune system dysfunction, inflammation, and autoimmune diseases such as lupus and multiple sclerosis (Ref.)
3) DNA demethylation (removal of methyl tags)
This is the main process taking place during the first stages of embryo development. It governs cellular programming which results in cell differentiation: the way stem cells are programmed to become tissue and organ-specific cells.
Certain DNA regions are turned on or off, and then modified via demethylation again for healthy development to take place (Ref.).
DNA demethylation can also play a role in the formation of tumors (Ref.) and this process is also taken advantage of in the design and use of drugs targeting different forms of cancer.
We have only very briefly touched upon this angle, however, hopefully, after being introduced to the main concepts behind methylation, as it relates to epigenetic modification, you can already notice that these processes are highly time-sensitive and require a deep dive into the individual health circumstances of each client. This topic is so vast that some of our staff spent 8 weeks attending an introductory course focusing on the role of methylation in controlling gene expression, on an epigenetic level.
It is our belief that while the Nutrigenomics community needs to start focusing on bridging the gap in understanding and explaining this distinction between these different angles of approaching the subject, likewise, the mainstream medicine professionals and writers should be giving more credit to the proactive approach and the initiative their nutritional therapeutics industry colleagues are taking, in using this knowledge to influence better lifestyle and dietary choices, with better targeted precision and tailored to each individual.
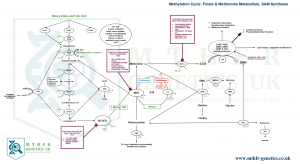
II. METHYLATION CYCLE (SAMe PRODUCTION)
The main focus in the world of Nutrigenomics, Naturopathics and Nutritional Therapeutics tends to be more on the methyl group production side of human biochemistry, rather than what happens after (as described in the first part of this article). As the MTHFR is the ‘gateway gene’ which kickstarts this process, the hype around it is somewhat understandable.
Methylation cycle as related to Folate metabolism has more of an impact on DNA production as it plays an important role in the synthesis of purines and pyrimidines.
The other, very important function of this cycle is the biochemical mechanism which results in SAMe (S-Adenosyl Methionine) production – our bodies’ chief methyl donor.
Role of SAMe as Methyl Donor:
- Allergies – by helping in histamine deactivation.
- Brain health, including mood (via deactivation of neurotransmitters serotonin and dopamine and also stress hormones: adrenaline and noradrenaline)
- Sleep – is used for Melatonin formation, out of Serotonin
- Cell membrane elasticity – Phosphatidylcholine production.
- Bile flow – through its role in choline formation. The methylation genes PEMT and BHMT are found in the liver and they make choline phospholipids, which are necessary to keep the bile flowing. (Ref). They can also prevent estrogen related cholestasis (Ref).
- Fertility and Hormonal balance – SAMe is used for catechol – Estrogen conversion, for example. This also ties in with Estrogen related cancers, where methylation may play a protective role. (Ref)
- Cancer prevention – to some extent with its role in producing methyl groups. These groups however have to be navigated properly, to ensure they silence DNA regions responsible for growth factors, which may help tumors grow. As we’ve learnt a few paragraphs above: over-methylation can also lead to cancers.
- Creatine formation.
- Donates methyl groups do DNA methyltransferases which makes DNA methylation (discussed above), possible.
The two aspects discussed in this article, are, of course, interconnected with each other : The Methylation Cycle (Folate, Cobalamin and Methionine metabolism) is geared towards the manufacturing process of the main methyl group donor: SAMe. The DNA Methylation, on the other hand, uses these methyl groups in order to modify the DNA behavior and to control genetic expression. We are, in the end, looking at the distinction between how many methyl groups are being produced vs. simply how are they being used, although there is nothing simple about the latter one.
This is a matter of the supply chain, vs. distribution and as demonstrated by this study, which found “No evidence for an association of MTHFR 677C>T and 1298A>C variants with placental DNA methylation” an issue with one, is not always necessarily synonymous with a potential issue with the other, when it comes to human biochemistry.
At the same time, we can not deny that our dietary habits may have a profound impact on the epigenetic control of gene expression. (Ref)
The overlap does exist, and the effort of closing the gap between these two concepts is perhaps most noticeable in the very big attention that the Naturopathic world is paying to a set of genes and enzymes connected to the Methylation cycle: SHMT BHMT, GNMT, PEMT, COMT.
As you can clearly notice, one thing these few genes have in common is the ‘MT’ part of their names. MT stands for Methyl Transferase and those are the type of genes which code for production of enzymes whose role it is to transfer a methyl group from a methyl donor (such as SAMe) or a methylated compound (such as Methylfolate) and attach it to other compounds in order to deactivate or degrade them.
From the naturopathic perspective this is the closest that we often get to touching on the mechanisms involved in moving methyl groups around, but a similar mechanism is also used in the DNA methylation processes described in the first part of this article.
While MT genes (Methyltransferases) are featured in ours and other Variant Reports, because of their specific roles in cycles responsible for reactions involving different types of folate, vitamin b12, homocysteine and methionine, phosphatidylcholine, creatine, dopamine, adrenaline and catechol-estrogens. DNA methylation works mainly by the action of DNMT genes (DNA Methyl-transferase genes) which regulate epigenetic modifications.
It is the DNA Methylation process that affects transcription, gene stability, and parental imprinting.(Ref.) It also directly impacts chromatin structure and can modulate gene transcription, or even completely silence or activate genes, without mutation to the gene itself.
It’s also these processes directly involved in regulating genetic expression which are associated with genetic disorders such as ICF, Rett syndrome, and Fragile X syndrome. (Ref)
A few words about MTHFR
Our modern food industry uses Folic Acid to fortify flour and other baked food products, under recommendations from public health organisations. The aim of this activity is to reduce the occurrence of neural tube defects.
Folic Acid has to be reduced into active forms in the liver and the capacity for this reduction seems to be only 250 micrograms/day (Ref).
This means that members of public who use fortified baked products and/or supplements with Folic Acid may end up with substantial levels of unmetabolised Folic Acid, in the bloodstream (Ref).
This may pose additional metabolic stress in the biochemistry of persons who already have decreased capacity for metabolising Folic Acid, as a result of genetic SNPs in their Folate Pathway.
In this expreiment, mice with homozygous MTHFR C677T variant, were fed high does of Folic Acid. The experiment concluded a significantly reduced MTHFR enzyme levels and activity as a result.
MTHFR and detox capacity
MTHFR has gotten a lot of attention, particularly when it comes to metal detox – It is true that there is a connection between MTHFR and Glutathione levels (cellular “master chelator” and antioxidant ) but not explaining the very complex mechanism of how heavy metals are really detoxed from human bodies, these kind of articles may incentivise a lay person to treat themselves with activated folate supplements or by taking glutathione or other chelating agents.
The only factor, by which we are directly able to verify the connection of MTHFR and Glutathione levels is the way that 5-MTHF (the product of the reaction mediated by the MTHFR gene) influence the expression of the GSS gene. GSS is responsible for assembling Glutathione from Glycine, Cysteine and Glutamate, and the methylated version of folate seems to promote the expression of this gene. Having higher Glutathione levels isn’t however enough and there are other dimensions to proper understanding whether an individual should be undergoing any detox protocols and most importantly to identifying the best time when a person would be ready for such protocol.
Please don’t take this article the wrong way: there is an appropriate time for using both of the above mentioned interventions, but it must be done strategically, and with taking into account many, many other factors if such therapy is to succeed without severe side effects.
The way human biochemistry deals with heavy metals, mycotoxins, organophosphates (from pesticides), xenobiotics etc. is a multistage, very complex and systemic process and any intervention with the aim of removal of these elements and compounds should ideally be designed to reflect and address each one of these stages: toxin conjugation, toxin removal from cells, movement of these conjugates inside the extracellular matrix and lymphatic system, excretion via bile, proteins involved in capturing and removal of toxic conjugates in kidneys and the intestines. Our Variant Reports list genetic variants connected to all of these stages.
There are companies out there, who, with their evaluation of your genetic results, will also issue a laundry list of supplements, to take or avoid. Often those same companies sell these products too. While it is educational to understand the kind of effect different compounds can have on specific genes, simply taking a supplement, based solely on SNPs results, will sooner or later backfire for most people choosing to go down that route. We’ve explained why this may be the case, in the DISCUSSION section, under the MTHFR SNPs description, in your variant report.
METHYLATION AND BRAIN HEALTH
Because of its’ role in regulating Homocysteine, the Methylation Cycle will have an impact on your brain health.
High Homocysteine can easily lead to an increase in Oxidative Stress.
Chronically increased Oxidative Stress goes hand in hand with inflammation.
Inflammatory cytokines and Reactive Oxygen Species can cause damage to the Blood-Brain Barrier which opens up access to the brain, for toxic and inflammatory compounds. This can be the starting point for one of many cascades leading to brain fog and cognitive decline (Ref) (Ref), (Ref).
High Oxidative stress due to increased Homocysteine will also impair the work of the mitochondria whose role is to manufacture ATP (energy).
Damaged mitochondria can lead to neurodegenerative diseases (Ref)
Increased Homocysteine may also contribute to the development of Alzheimer’s and Parkinson’s disease due to damaging effects homocysteine can have on glial cells and neurons (Ref. )
Higher levels of Homocysteine can worsen mood, cause trouble with cognition and promote anxiety. (Ref.), (Ref.) This seems to be connected to Homocysteine’s impact on the Glutamate – GABA balance.
Homocysteine stimulates NMDA receptors which in turn increases Glutamate production and activity in the brain. Glutamate is an excitatory neurotransmitter which, when in excess, can significantly contribute to levels of anxiety.
In addition to that, Homocysteine may also lower GABA – the inhibitory neurotransmitter responsible for the feeling of relaxation. (Ref)
Methylation and its product SAMe is vital to metabolism of some of your predominant neurotransmitters: Dopamine, Adrenaline and Noradrenaline.
The COMT gene and its’ product COMT enzyme, manages the levels of these neurotransmitters which govern stress recovery, learning abilities and social interactions abilities.
Too much dopamine can lead to aggression, psychosis, and low emotional intelligence (Ref), (Ref), (Ref)
Poor methylation function can increase the risk of cognitive and mental health problems (Ref)
Levels of active forms of folates are the gateway of the Methylation Cycle and folate deficiency may be contributory factors of many mental illnesses, including schizophrenia, depression, autism, and bipolar disorder.
Folate deficiency has been discovered in close to one-third of the people with depression. (Ref)
The Folate Cycle is responsible for the formation of new brain cells, which supports brain development in children and learning in adults.
METHYLATION AND REPRODUCTIVE HEALTH
The last step in producing the most bio-active form of folate is the action of the MTHFR gene. This form of folate also donates methyl groups to the rest of the Methylation Cycle which controls levels of Homocysteine. (Ref.)
High Homocysteine levels and low SAMe lowers fertility and reduces the chance of conception and a successful, healthy pregnancy because of inflammation and oxidative stress. (Ref), (Ref)
Metabolically active forms of folate are vital for the building process of DNA and RNA structures and thus are necessary for DNA repair. When DNA is “repaired” without folates, it most likely results in serious mutations. Folates, therefore, protect your genetic material and will increase your chance of successful pregnancy. Low levels of folate contribute to reduced fertility and increase the chance of birth defects and miscarriage because of damaged DNA material being passed on (Ref), (Ref.)
Methylation Cycle , Folate Cycle and Conception
Healthy egg follicles, as well as the lining of the uterine, heavily depends on the ability of cells to divide and grow – a process that is controlled by the folate cycle.
Eggs release is necessary for the ovulation to take place and there can be no natural conception without ovulation and the sperm needs to able able to revitalise an egg.
This study shows that women who didn’t ovulate had higher homocysteine levels (Ref).
If poor methylation leads to increased levels of Homocysteine and high Homocysteine indeed interferes with egg ripening and ovulation, then poor methylation may as well be considered as a serious factor contributing to difficulties with the ability to get pregnant.
Poor methylation may also lower hormones important for fertility, such as progesterone which plays a very important role during the luteal phase (the last 14 days of women’s’ monthly cycle).
Progesterone makes it possible to the embryo to be implanted inside the uterine lining – a process which marks the start of every natural pregnancy (Ref).
It has been found that women who have high blood folate levels also have higher luteal phase progesterone and thus are more fertile (Ref).
It may, therefore, be assumed that Folate deficiency might underlie low progesterone in some women who don’t ovulate and have trouble conceiving.
Among women undergoing fertility treatments, those with “bad” MTHFR variants (C677T mutation) produced less estrogen.
Women with homozygous MTHFR 677 variants, who were undergoing fertility treatments were found to produce less estrogen.
They were also shown to be less responsive to ovulation triggering hormones such as FSH (follicle-stimulating hormone) (Ref.), (Ref.)
Estrogen is very important for the growth of the uterine lining which needs to be thick, in order to provide enough blood and nutrients, to sustain pregnancy and ensure successful fetal development.
Thin uterine also lessens the chance of egg implantation.
During pregnancy, women require more folate to grow the fetus and placenta.
Growing fetus and placenta require more folate, however, during pregnancy, women absorb less of this vitamin. (Ref.)
Low folate and subsequent high Homocysteine and compromised methylation cycle, contribute to the risk of miscarriages, Neural Tube Defects, low birth weight and overall pregnancy complications. (Ref.), (Ref) , (Ref.), (Ref.)
Methylation and Menopause
Women post-menopause have typically higher levels of Homocysteine which points to the poorer function of the Methylation Cycle. (Ref), (Ref), (Ref), (Ref)
Menopausal women with folate deficiency have a lower bone mineral density (Ref.), (Ref.)
Folate may also help reduce hot flushes in some menopausal women (Ref.)
We can, therefore, assume that postmenopausal women have a higher need for Methylation Cycle support and Folate intake.
Methylation & Male Reproductive Health
High Homocysteine can reduce blood flow as it lowers Nitric Oxide. Poorer blood flow has been identified as the culprit behind erectile dysfunction. (Ref.)(Ref).
Controlling Homocysteine levels can improve blood flow which in turn can help to prevent erectile dysfunction (Ref.)
One study done on mice found that poor methylation and removal of the MTHFR gene function results in the production of unhealthy sperm and overall infertility. (Ref)
Another study demonstrates that men with homozygous MTHFR 677 variants are more prone to infertility, due to problems with Homocysteine metabolism – this also points to dysfunctional Methylation Cycle. (Ref.)
Healthy sperm and its’ production needs adequate amounts of folate. As folate in sperm increases, it becomes more dense and higher in spermatozoids which makes it much more fertile. (Ref.)
Men without an SNP in the MTHFR677 position had increased semen density. (Ref.)
METHYLATION, IMMUNE FUNCTION AND INFLAMMATION
Compromised efficiency of the Methylation Cycle can lead to raised levels of Homocysteine which increases inflammation and oxidative stress. (Ref), (Ref), (Ref)
Properly supported Folate intake and the addressing of Methylation and Trans-sulfuration cycles can balance the immune system by regulating the levels of Treg cells and Th17 inflammatory cytokines. Th17 dominance is often a hallmark of autoimmune diseases. (Ref)
Folate intake has been found to reduce inflammation in immune cells with high Homocysteine. (Ref), (Ref)
Pregnant women who supplemented with higher doses of synthetic Folic Acid were more likely to have children who developed asthma and allergies in their early childhood. This was especially true for mothers with MTHFR C677T SNPs. (Ref), (Ref)
On the other hand, children with Asthma, who followed a diet high in methyl donors, like folate, had fewer symptoms. (Ref)
ADDRESSING METHYLATION
Before your Practitioner decides to fully and directly address your Methylation Cycle (including the MTHFR SNPs) you should discuss, assess and consider addressing all of the areas which put additional stress on this part of your biochemistry:
- GI Health
- Oxidative Stress
- Inflammation
- Cell membrane fluidity
- Mitochondrial Function
Knowing about your MTHFR SNPs beforehand, however, is not without its benefits, but again – not on its own.
One of the first labs people may want to run after identifying MTHFR SNPs is measuring Homocysteine levels.
A slowed down methylation pathway can lead to raised Homocysteine – an inflammatory amino acid. A buildup of Homocysteine can lead to high levels of free radicals (oxidative stress) and increased rates of cell death (apoptosis), which then can lead to tissue damage, immune system problems and even autoimmunity (Ref, Ref, Ref).
There have been several significant studies done on rats and their results linked high homocysteine levels to higher intestinal barrier permeability (aka leaky gut) (Ref. ), (Ref.)
On the other hand, we also know that folate metabolism is absolutely crucial for cellular growth and division and thus plays an important role in tissue regeneration. By addressing the methylation cycle properly we can achieve two things at the same time: lower the level of inflammation (and subsequent tissue damage) and aid our body’s ability to heal itself.
Having identified these potential issues, to which MTHFR can be connected, does not automatically mean that one should start tackling them by necessarily addressing MTHFR directly and as the first point of action – unfortunately our bodies are far more complicated and it’s not that easy. This knowledge about genetic predispositions or variants, in combination with other factors and keeping in mind metabolic interactions, downstream effects and upstream influences, can help in identifying the level of support as well as the best timing for addressing any given SNP and the entire related pathways.
There’s more to raised Homocysteine than just MTHFR. Other genes like BHMT, MTR and MTRR as well as AHCY, are also involved. It is therefore quite important to adopt a different mindset and to look at these polymorphisms in the context of entire pathways, while also taking into consideration environmental factors, like diet and exposure. Shifting your understanding to such an approach will inevitably lead you to looking at particular SNPs not as the cause behind your ailments, but rather as contributory factors.
You may have already noticed that Gut Health and Inflammation are the first two sections on our reports and it’s also one of the first stages to address, as depicted on our Functional Healing Planner and its strategies designed to address these areas, that we would recommend focusing on first. For example, treating acetaldehyde producing yeast infections (like candida) should either come before or be applied simultaneously with repairing of the gut tissue. Acetaldehydes can have an inhibitory action on the MTR gene and thus can contribute to raised Homocysteine levels. It would be logical to remove or reduce the cause behind the tissue damage first (like inflammation and pathogens causing inflammation), before attempting the repair protocols.
Supplementation
Taking moderate doses of methyl folate or other methyl donors as supplements, when there are no existing, chronic health issues, and simply as a form of prevention and good health maintenance is in itself a different topic and such regimen could potentially have many health benefits, especially for people living in areas where access to green leafy vegetables isn’t that easy all year around – although nowadays this would be highly unlikely.
Please also read the INTRODUCTION to your Variant Report.
